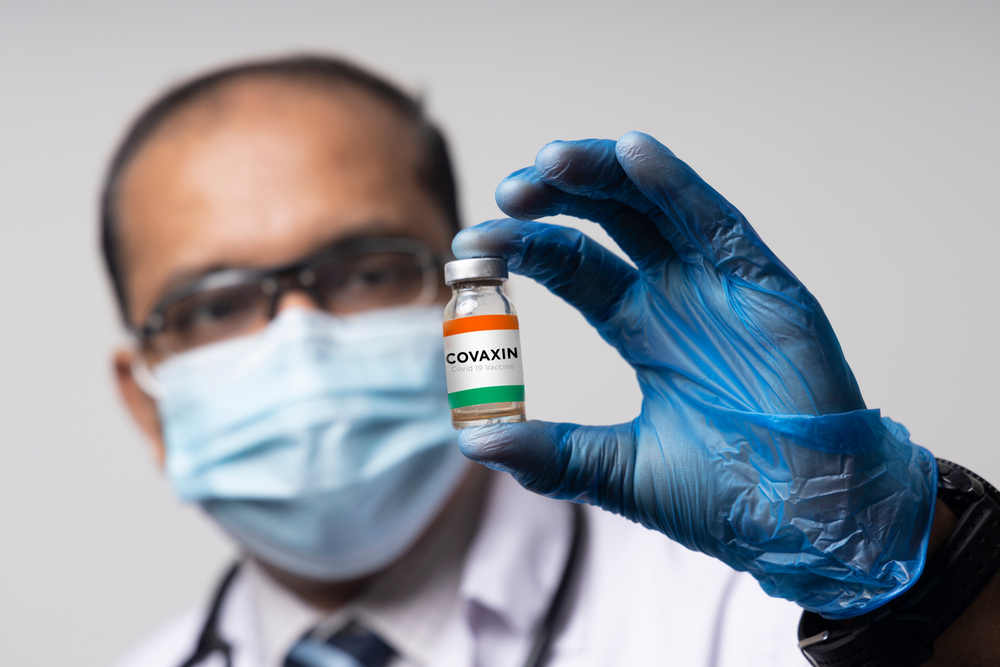
São Paulo’s flagship Albert Einstein Hospital will carry out late-stage clinical trials on the Indian-developed coronavirus vaccine Covaxin, despite health regulators deciding that producers of Covid-19 immunizers no longer need to hold phase 3 trials in the country to qualify for emergency approval.
Starting in March, the trials should last for up to 90 days. As regulators have committed to analyze approval requests for vaccines tested in other countries within 30 days, there is a chance the immunizer could be cleared for use before Brazilian tests are concluded. The federal government is keen on purchasing 30 million doses of Covaxin and the Russian-made Sputnik V, which has also not undergone clinical trials in Brazil.
However, the Albert Einstein Hospital believes trials must continue in the country as a way to boost local research and speed up immunization coverage. “[By performing trials,] you expand clinical research in Brazil and the participants are given shots. So you can reduce the cost of immunization to the country and vaccinate people faster,” said Luiz Vicente Rizzo, the hospital’s director, to news website G1.
 Support this coverage →
Support this coverage →
 Search
Search