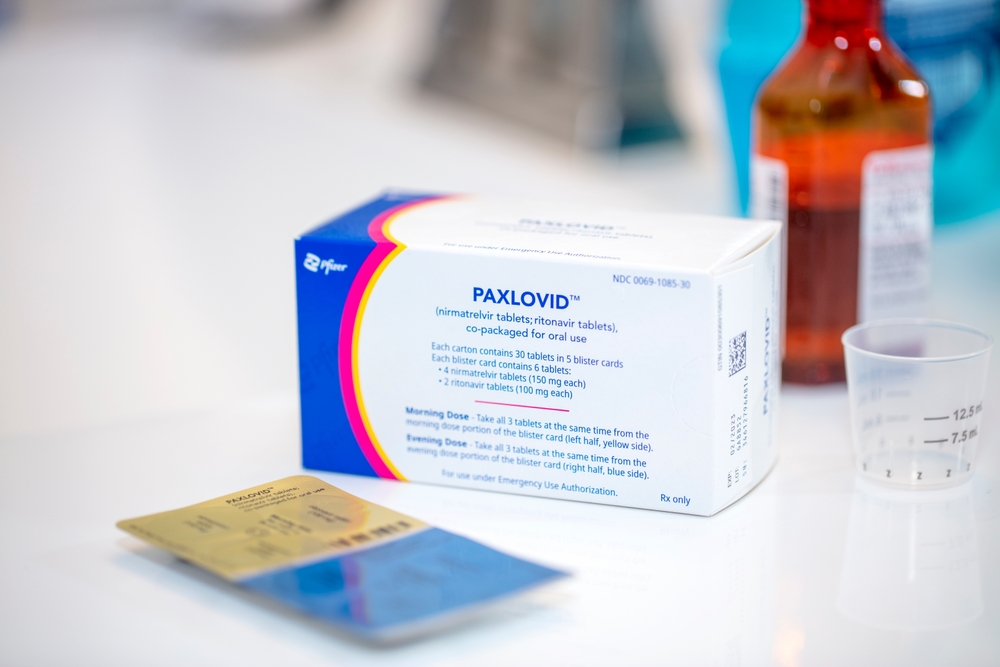
Anvisa, Brazil’s federal health regulatory agency, on Monday cleared the sale of Pfizer’s pills for treating Covid. The drug, sold under the brand name Paxlovid, will require a doctor’s prescription — and Pfizer must observe provision requirements to the public healthcare system.
Regulators also extended Paxlovid’s expiration date to 18 months after production instead of 12 months.
In a statement, Anvisa said its decision to facilitate access to the Covid drug was influenced by the current epidemiological situation in Brazil, “with the spread of new Omicron subvariants and the rise of case numbers.” The country’s seven-day rolling average of new Covid cases has quadrupled in the seven days to Sunday, hitting 15,500. The data doesn’t include figures from six states.
Pfizer obtained emergency-use authorization to sell Paxlovid back in March of this year. The drug gained approval from the U.S. Food and Drug Administration on December 22, 2021, for patients aged 12 and over.
Meiruze Freitas, an Anvisa board member and the case’s rapporteur, said Paxlovid sales in the private market will increase access to Covid treatments, since the drug must be taken within five days after the onset of symptoms.
“Early diagnosis and hospital care, when necessary, are important to prevent the disease from progressing into severe cases,” she told her peers. Ms. Freitas also reiterated that treatment does not replace vaccination. “Vaccination remains the best strategy to avoid Covid, hospitalizations, and deaths.”
Over 80 percent of the Brazilian population has completed its first vaccination cycle. These high rates came despite the government’s conscious efforts to stall rollouts (which included the president stalling on vaccine deals and spreading misinformation on its possible side-effects).
Only half of the country has taken booster shots, and rollouts for children have encountered many hiccups.
A study published on Monday in The Lancet Regional Health Americas journal finds that “an additional 47,000 lives could have been saved had the Brazilian government started the vaccination program earlier.”

 Search
Search