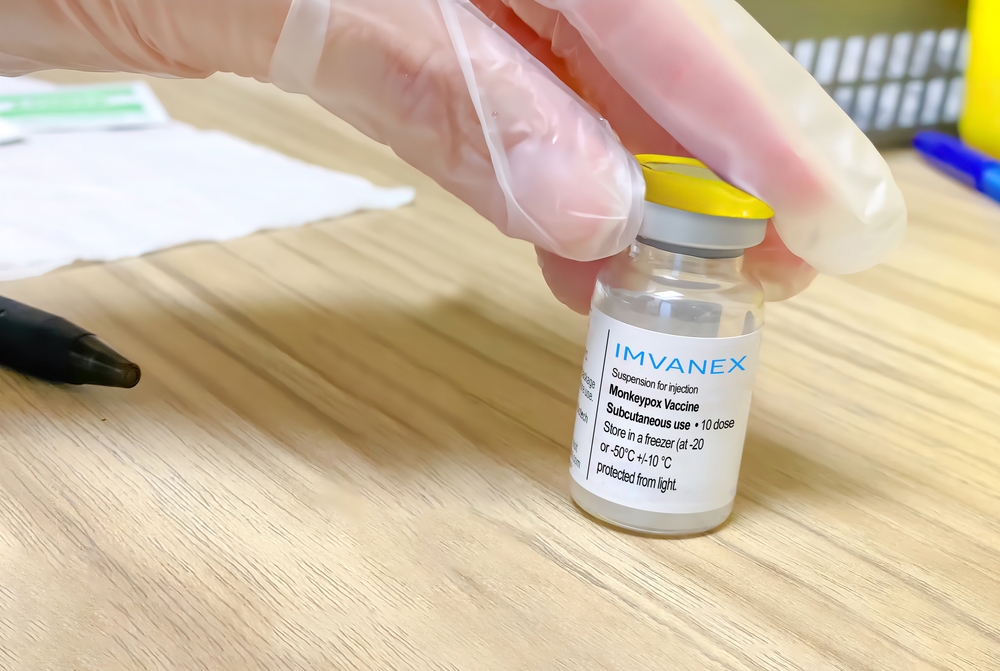
Brazil’s federal health regulator Anvisa announced today that it approved a request by the Health Ministry authorizing the use of the Jynneos/Imvanex monkeypox vaccine in Brazil.
The green light from Anvisa is valid for six months and was based on analyses by the U.S. Food and Drug Administration, the European Medicines Agency, and the United Kingdom’s National Health Service. Still, the case’s rapporteur discussed the need for monitoring to further confirm the vaccine’s efficacy against monkeypox.
The Jynneos/Imvanex vaccine is produced by Danish pharmaceutical firm Bavarian Nordic. Originally developed for smallpox, it has been the most widely used against monkeypox. Due to its limited supplies, health officials worldwide have limited the rollout to healthcare workers and people who have suspected or confirmed exposure.
A week ago, Anvisa had already passed a new regulatory framework to speed up the approval of monkeypox vaccines and drugs. Through the Pan American Health Organization, Brazil expects to receive 50,000 doses this year, to be delivered in two separate batches.
Brazil has been one of the world’s monkeypox hotbeds, with over 4,200 confirmed cases (with another 4,600 suspected infections). Only the U.S. (16,800-plus) and Spain (almost 6,300) have higher tallies. Experts, however, warn that the real numbers might be massively underestimated.
The World Health Organization reported that the outbreak has lost steam in Europe. However, “the lack of public health measures” in Latin America, combined with the lack of vaccine doses, allows the disease to further spread.

 Search
Search