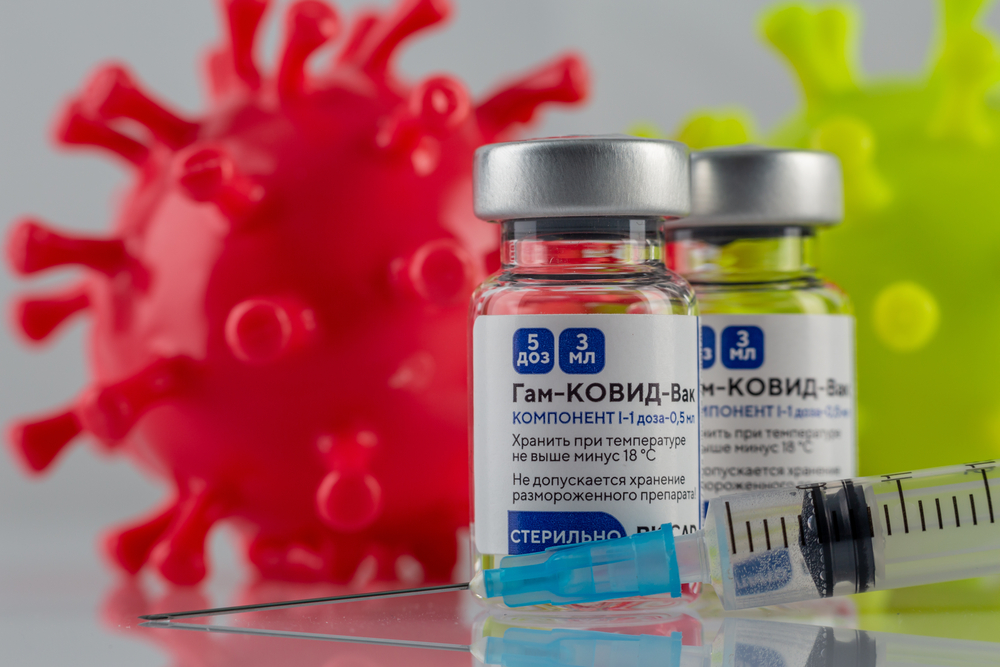
The board of Brazilian health regulator Anvisa rejected requests to import the Russian-made Sputnik V vaccine. The unanimous decision came after the agency’s technical departments identified problems asserting the vaccine’s quality and safety.
The decision refers to a request for emergency approval submitted by ten states. Anvisa will evaluate another six such requests, issued by four states and two municipalities. If the Sputnik V producers are able to submit further information about the immunizer, Anvisa could reconsider its ruling.
On Twitter, the producers of Sputnik V said they handed over more information to Anvisa than to any other agency in the 61 countries which greenlit the use of the vaccine. “We hope science, and not pressures from another country, will base such decisions,” they said.
A Sputnik V compartilhou com a Anvisa todas as informações e documentações necessárias, muito mais do que o utilizado para homologar a Sputnik V em 61 países. Esperamos que a ciência, e não a pressão de outro país, seja usada para a tomada de decisão.
— Sputnik V (@sputnikvaccine) April 26, 2021
👇https://t.co/V8XgTrXcWw
The tweet was a reference to pressure by the Donald Trump administration to convince Brazil not to purchase Sputnik V, out of fear of enhanced Russian influence in Latin America. The 2020 Annual Report of the U.S. Department of Health and Human Services (HHS) includes one bullet point about “Combatting malignant influences in the Americas”:
OGA [The Office of Global Affairs] used diplomatic relations in the Americas region to mitigate efforts by states, including Cuba, Venezuela, and Russia, who are working to increase their influence in the region to the detriment of US safety and security. OGA coordinated with other U.S. government agencies to strengthen diplomatic ties and offer technical and humanitarian assistance to dissuade countries in the region from accepting aid from these ill-intentioned states. Examples include using OGA’s Health Attaché office to persuade Brazil to reject the Russian COVID-19 vaccine, and offering CDC technical assistance in lieu of Panama accepting an offer of Cuban doctors.

 Search
Search






































