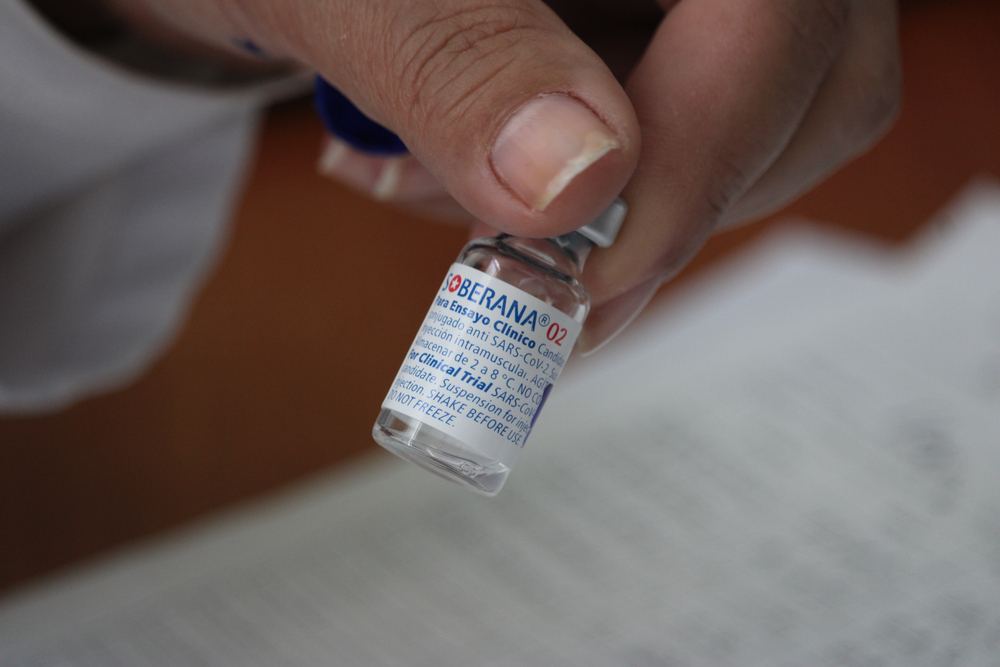
Health authorities in Iran announced they will begin phase-three clinical trials on the Cuban-made Soberana 02 vaccine, one of the five immunizers developed by the island nation and the only fully made-in-Latin-America vaccine so far.
The trials will involve 24,000 Iranian volunteers and eight different universities, said Alireza Biglarí, director of Iran’s Pasteur Institute. In two of the eight departments that will be part of the trial, a booster dose will be given in addition to the two originally recommended shots, as it “improves immunity of people that were already infected,” Mr. Biglarí said.
Cuba is close to becoming the smallest country in the world to approve its own homemade Covid-19 vaccine. The 11-million-people nation plans to produce over 100 million doses for domestic use and send the surplus to allied countries such as Vietnam and Venezuela.
Though the vaccination campaign in the island has not yet begun, more than 125,000 people have received doses during trials. Around 44,000 volunteers received two vaccine doses.
Cuba had largely been able to control the coronavirus, but the island nation is now in its worst period of the pandemic, registering more than 1,000 new cases per day for the first time. As of April 20, total cases topped 94,571 and 531 people have died from Covid-19.

 Search
Search